Live Science is your one-stop shop for the latest discoveries, groundbreaking research, and fascinating...
The UK National Protective Security Authority (NPSA) has recognized the Israeli counter-drone company, Sentrycs,...
TSMC has announced its latest 1.6nm technology, A16, at the 2024 North America Technology...
The Xpeng G9 has recently caught the attention of many onlookers at a mall...
On Saturday, New Ulm baseball was victorious against Worthington. Luke Suess played a pivotal...
The first group of employees at Van Hool’s trailer division is expected to return...
On April 19, 2024, a pilot study published in the Journal of Neurosurgery: Spine...
A recent study by ACCA and IMA reveals that finance professionals are feeling optimistic...
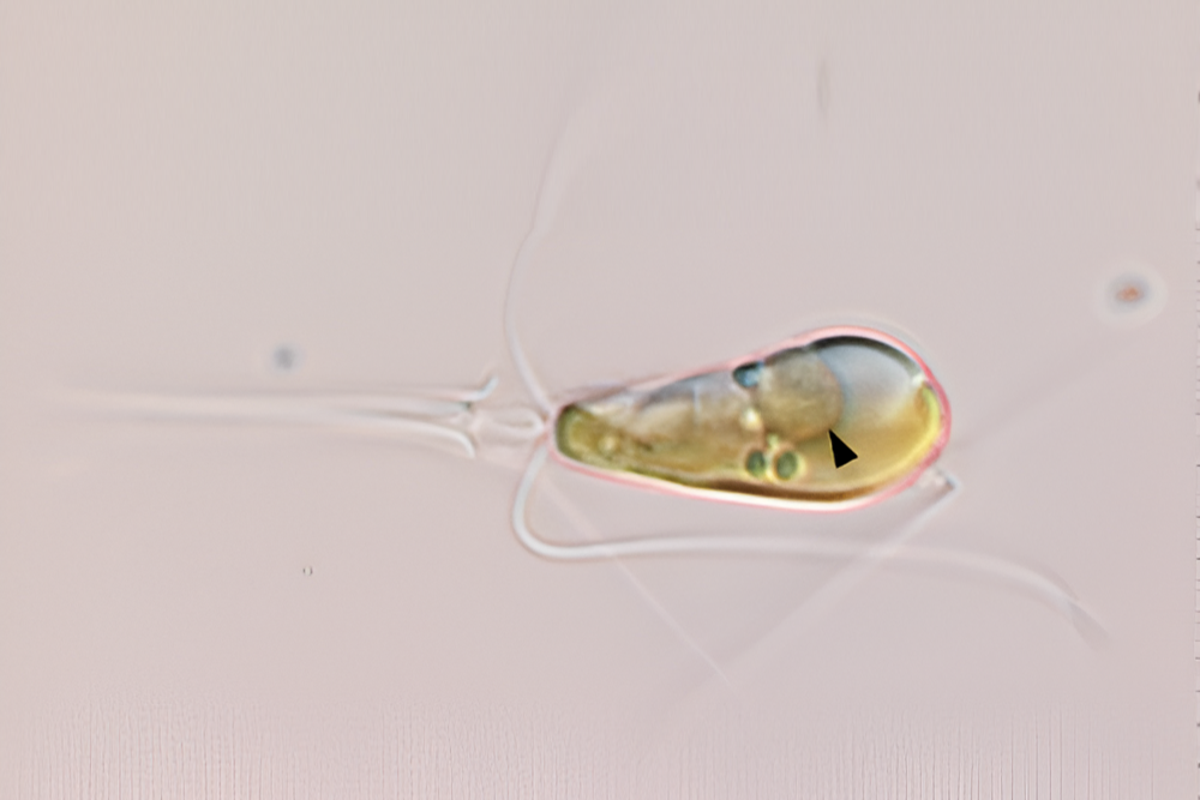
In a groundbreaking evolutionary event, two lifeforms have merged to form a single organism...
German politicians and business leaders are increasingly discussing a topic that was previously considered...